Children’s Intelligence Syrup has a market size of 50 million yuan, and Sunflower Pharmaceutical’s products account for 70%.
Special medicine or IQ tax? Sunflower Pharmaceutical Children’s Intelligence Syrup is questioned
Blue Whale News, February 21 (Reporter Tu Jun)“Intelligence syrup for treating ADHD and autism? I feel that those who believe in this need to treat their intelligence.& rdquo;
“Today, when I went to the hospital, the doctor prescribed this medicine for my child and asked me to go to an outside pharmacy to buy it. At the same time, he told me to remember to give him some psychological hints after drinking it to make me think it should be a placebo. rdquo;
Recently, a proprietary Chinese medicine pediatric intelligence syrup has attracted attention. According to the website of the State Food and Drug Administration, there are currently 10 approval numbers for pediatric intelligence syrup, including: Sunflower Pharmaceutical Group Chongqing Xiaoxuihua Children’s Pharmaceutical Co., Ltd., Taiji Group Chongqing Fuling Pharmaceutical Factory Co., Ltd., China Resources Sanjiu (Tangshan) Pharmaceutical Co., Ltd., etc.
As a prescription drug, what is the clinical basis for pediatric intelligence syrup to improve intelligence? Recently, Blue Whale Finance tried to contact Sunflower Pharmaceutical, Taiji Group and other companies, but did not receive a clear reply.
How long it will work and how much clinical evidence will support it is questionable
According to the website of the State Food and Drug Administration, there are 10 approval numbers for pediatric intelligence syrup, involving 6 manufacturers. Among them, Taiji Group Chongqing Fuling Pharmaceutical Factory Co., Ltd. has 5 approval numbers, and the other 5 manufacturers are China Resources Sanjiu (Tangshan) Pharmaceutical Co., Ltd., Sunflower Pharmaceutical Group Chongqing Xiaokuihua Children’s Pharmaceutical Co., Ltd., Hunan Xinhui Pharmaceutical Co., Ltd., Shanghai Baolong Anqing Pharmaceutical Co., Ltd., Luoyang Shunshi Pharmaceutical Co., Ltd.
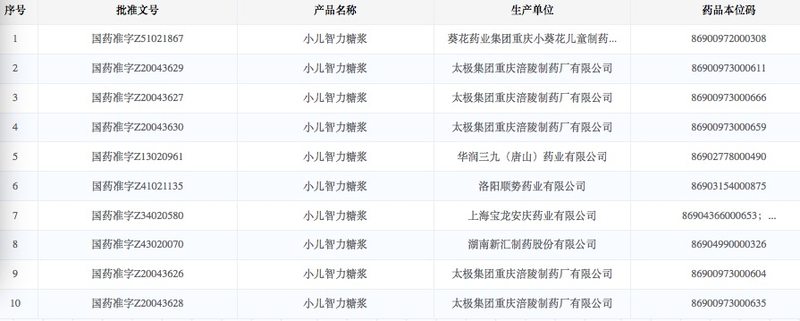
Photo source: State Drug Administration official website
Blue Whale Finance found that although it was produced by different manufacturers, the ingredients were all the same, including: turtle shell, dragon bone, polygala, sweetflag, and rooster. The preparation document numbers were all Chinese medicine Z, representing proprietary Chinese medicines.
Regarding whether children’s intelligence syrup can improve children’s intelligence, Lanjing Finance called Sunflower Pharmaceutical’s after-sales service hotline. The other party said that the drug can indeed improve children’s intelligence. The principle is that Acorus gramineus can regulate neurotransmitters in the central nervous system; Polygala, which has the effect of activating blood circulation and removing blood stasis, improving blood circulation in the brain; and tortoise shell, which can calm and soothe the nerves. In addition, it said that the drug had undergone clinical trials, but when asked about the specific trial content and results, it said that the problem was beyond the scope.
The above-mentioned staff did not give a clear reply on how long it would take for the child to take effect, and the drug instructions did not clearly state it.
A doctor from a top-three traditional Chinese medicine hospital told Lanjing Finance that whether it is to strengthen memory or promote intellectual development, the process of children’s development is an overall process and cannot be solved by a few medicines. In addition, traditional Chinese medicine and western medicine are two systems. Western medicine emphasizes evidence-based medicine and provides the right medicine.
Blue Whale Finance also called Taiji Group’s investor hotline and other relevant telephone numbers, but the other party did not give a clear reply.
Public information shows that the prescriptions of tortoise shell, dragon bone, polygala tenuifolia, sweetflag, and rooster are derived from the Kong Sheng Zhenzhong Dan in “Qianjin Prescription”, and are endowed with the effects of regulating, nourishing the heart, kidney, tranquilizing the nerves, and improving the intelligence.
In 1982, Qu Xiuhua, chief physician of Longhua Hospital Affiliated to Shanghai College of Traditional Chinese Medicine, in clinical practice, referred to Kong Shengzhen Zhongdan as the main prescription and added rooster juice to make pediatric intelligence syrup. Qu Xiuhua pointed out in a paper entitled “Traditional Chinese Medicine Intelligence Syrup for the Treatment of 100 Cases of Minor Brain Dysfunction Syndrome in Children” that the researchers selected 100 children for the test, claiming that 70 children had significant improvement in their symptoms 24 and 72 hours after taking the drug. They listened attentively in class, no longer made small movements, and had excellent test scores during taking the drug. Based on this, it is believed that the drug is effective in children with MBD, which is also the main basis for the 1993 version of the drug standard to include MBD as indications.
However, the author also admitted in the paper that whether intelligence syrup can increase the number of neurotransmitters and enhance self-control needs further research. During taking the medicine, MBD children should pay attention to strengthening their individual education and cannot rely solely on taking the medicine. rdquo;
The Minimal Brain Dysfunction (MBD)mentioned in the article is a general term for hyperactivity, impulsiveness, inattention, learning difficulties, etc. before attention deficit hyperactivity disorder (ADHD) became the official diagnostic name.
In fact, on social platforms, people have mixed reviews of pediatric intelligence syrup. Some doctors have recommended it as a commonly used drug for ADHD. However, doctors from many Shanghai Children’s Hospitals consulted by Blue Whale Financial said they did not understand this drug. Some doctors said that the lack of use is related to the fact that this drug is not included in medical insurance.
However, although this drug has not entered the national medical insurance catalog, it has entered some local medical insurance catalogs. In 2020, the National Medical Insurance Administration required all localities to clear excess drugs within three years, only implement the National Medical Insurance Catalogue, and set a three-year transition period, resulting in the withdrawal of pediatric intelligence syrup from the medical insurance market.
In terms of clinical data, in fact, there is almost no public clinical data information on pediatric intelligence syrup on the official website of the company with approval number. Whether large-scale clinical trials have been conducted has been questioned.
Is it necessary to conduct clinical trials for proprietary Chinese medicines? Lawyer Gui Xin, co-partner of Beijing Deheheng (Shanghai) Law Firm, and co-founder of Tianmu Life Sciences Legal Team, told Lanjing Finance that in general, most traditional Chinese medicines need to conduct clinical trials in strict accordance with laws and regulations, but some Chinese medicine countries have also opened green channels. According to the “Special Provisions on the Registration and Administration of Traditional Chinese Medicine”, traditional Chinese medicines that are urgently needed under some specific conditions may be applied for listing without conducting clinical trials.
According to the “Regulations on the Administration of Simplified Registration and Approval of Ancient Classic and Famous Formulations of Traditional Chinese Medicine Compound Preparations”, classic and famous formulations that meet its requirements may be applied for marketing without conducting clinical research. In addition, for traditional oral proprietary Chinese medicines already marketed in Hong Kong and Macao, in accordance with the “Announcement of the State Food and Drug Administration on Simplifying the Registration and Approval of Traditional Oral Chinese Medicines already marketed in Hong Kong and Macao (No. 7 of 2025)”, application for listing in the Mainland can be exempted from clinical trials.
“Although exemption from clinical trials saves pharmaceutical companies a lot of time and money, it requires providing a lot of supporting materials. Moreover, whether the drug’s marketing application can be approved is the final review by the State Food and Drug Administration. From a practical perspective, it is not an easy path. rdquo; Lawyer Gui Xin pointed out.
With a market size of 50 million yuan, Sunflower Pharmaceutical accounts for 70%
The price of a box of pediatric intelligence syrup on the e-commerce platform is about 30-40 yuan, containing 10 vials, each of 10ml, three times a day, with 10-15ml each time. Based on this calculation, the monthly cost of children is about 270-540 yuan.
Data shows that about 70% of pediatric intelligence syrup is sold through urban public hospitals, followed by urban pharmacies, accounting for about 10%. Among them, the average sales of the head manufacturer of this drug in the past five years have exceeded 50 million yuan. Sunflower Pharmaceutical has a high market share of pediatric intelligence syrup, accounting for about 70%, followed by Shanghai Baolong Anqing Pharmaceutical. From 2019 to 2023, Sunflower Pharmaceutical’s sales of pediatric intelligence syrup have a trend of decline and then rebound, which are 66.95 million yuan, 57.26 million yuan, 52.87 million yuan, 46.51 million yuan, and 54.41 million yuan respectively, and 31.01 million yuan in the first half of 2024.
In terms of performance, according to Sunflower Pharmaceutical’s 2024 performance forecast, net profit in 2024 is expected to drop by 41.03%-60.68%, to approximately 440 million to 660 million yuan. Not only Sunflower Pharmaceutical, according to Wind Financial Terminal statistics, a total of 30 listed companies in the Chinese pharmaceutical sector have issued performance forecasts, of which only 8 companies have positive net profit changes.
Some industry insiders said that due to policy dividends and other factors, the traditional Chinese medicine sector has been counter-current in the cold winter of medicine. However, as the policy dividends are gradually disappearing, demand-side users have begun to pay more attention to the specific clinical value of drugs, and the controversy over the efficacy of proprietary Chinese medicines is harmful to the overall market. Although the market for Xiaoer Intelligence Syrup is only 50 million yuan, its problem seems to be of universal significance, and it may be clear that the efficacy is the first step needed to standardize traditional Chinese medicine.
On July 5 last year, the State Food and Drug Administration announced that the “Special Provisions on the Management of Traditional Chinese Medicine Standards” had been reviewed and approved on July 4 and will come into effect on January 1, 2025. This regulation aims to build a strict drug standard system, combine generic requirements with the characteristics of traditional Chinese medicines, clearly define the research, formulation, revision and implementation of traditional Chinese medicine standards, clarify the responsible entities, and standardize key processes.
The formulation of the “Special Provisions on the Management of Traditional Chinese Medicine Standards” aims to solve problems such as inconsistent quality and inconsistent standards in the traditional Chinese medicine industry. After the implementation of the regulations, it is expected that the quality standards of traditional Chinese medicines will be improved, medication safety will be ensured, and the standardized development of the traditional Chinese medicine industry will be promoted. The State Food and Drug Administration plans to carry out publicity and implementation training to improve the ability and level of standard management of traditional Chinese medicine.



